|
Daynotes
Journal
Week of 17 March 2008
Latest
Update: Friday, 21 March 2008 08:22 -0500 |
08:57
-
Our friends Paul Jones and Mary Chervenak came over yesterday. Paul and
Barbara watched the basketball game upstairs while Mary and I shot some
video in the downstairs lab. We ended up with nearly an hour of raw
video, which will be edited into a 10-minute video that I'll post to
YouTube.
This was pretty much a trial run. As expected, the
audio gave us problems, but those shouldn't be too hard to fix. For the
talking-head intro sequence, Mary will just re-record the audio,
lip-syncing to the video. Most of the time, the camera is pointed at
the reaction vessel, and Mary is off-camera except for her hands. She
can re-record the audio for those segments as a voiceover.
I'm
less than delighted with the color rendering. I did set a manual white
balance by pointing the camera at a white sheet of paper and telling it
to set a custom white balance, but it's still obvious that this footage
was shot under fluorescent lighting. Still, I think it's okay for what
we want to do.
I've been exchanging emails with a high-school chemistry teacher in
Ohio who, with the aid of a grant, has developed a forensics course for
her students. She tells me that there aren't any good forensics lab
books available for such a course, which I already knew.
But her
initial excitement about my book was dampened when she looked at the
list of chemicals I specified for the home chemistry book. Many of
those chemicals, she tells me, are banned by the state of Ohio for use
in schools because they're too dangerous for students to use. That
concept of "too dangerous" is an interesting one to explore.
Many
state and federal organizations have published listed of chemicals that
they recommend not be used in high-school labs, or that they recommend
be used only with restrictions. Here's one such list
(PDF), published by North Carolina in the late 1970s. It's a complete
mish-mash. Some of the chemicals listed are indeed quite hazardous, but
many others are no more hazardous than chemicals routinely found in
bathrooms, kitchens, and basements across America. Others present no
real danger at all. And many of the most hazardous chemicals routinely
used in chemistry labs, such as sodium hydroxide, don't even make the
list.
Here's a message I sent to my friends Paul and Mary after I read the list:
From: Robert Bruce Thompson
To: Mary Chervenak, Paul Jones
Date: Fri Mar 14 16:27:36 2008
Re: Chemicals, oh my
Geez.
These North Carolina laboratory safety guidelines for schools are a
real hoot. Among the chemicals they recommend never be used in a school
lab are chromium (chunks of the metal are apparently deadly; potassium
dichromate isn't listed as dangerous) and hydrogen, not to mention the
deadly methyl orange and methyl red.
And
then there's carbon dioxide, which requires great care because inhaling
it can make you lose consciousness and die. But my favorite of the
deadly dangerous chemicals they list is ... tannic acid. That, and the
mystery dangerous chemical described only as "Magenta", which I thought
was a color.
They
also say in the biology section that poinsettia is lethal. One leaf can
kill a child, says they. And here I thought poinsettia was nontoxic.
They make up for it, though. They point out that monkshood can cause
"digestive upset and nervous excitement". Duh. Given that all parts of
the monkshood plant contain aconite and aconitine, which is lethal in
tiny quantities, and that adults have died as a result of eating only a
small amount of the plant, I'd say that I'd certainly suffer "digestive
upset and nervous excitement" if I ate any. Right before I died, that
is.
Geez.
All of these publications claim that the top priority is the safety of
students, and all of them lie. If safety is truly your top priority,
you avoid doing anything at all that involves any risk. If the safety
of students were really the top priority, they wouldn't have chemistry
labs at all, because any work in a chemistry lab involves some risk, no
matter how small. The top priority is teaching the students something
about chemistry. Student safety, by definition, can be at most the
second priority.
What they're really saying, of course, is that
lab activities are important enough to make some risk acceptable, and
that within that acceptable level of risk, student safety is
the highest priority after the priority of doing the lab sessions
themselves. It's a question of accepting a certain level of risk in exchange for an educational benefit.
Can kids get hurt in a chemistry lab, whether at
home or at school? Sure they can. Deaths and serious injuries, while
very rare, do happen. Nearly every time something bad happens, it's
because someone did something incredibly stupid. But bad things can
happen even if you follow all the rules and take every safety
precaution.
But merely because there are risks involved in a useful
activity is no reason not to engage in that activity. Life is
dangerous. You can die without getting out of bed. You can be struck by
lightning while you're walking your dog. You can be killed in a car
accident through no fault of your own on your way to the grocery store.
But people continue walking their dogs, driving to the grocery store,
and doing other "dangerous" things because the alternative is to do nothing at all.
I
think I'm going to buy a new printer. When I was printing copies of the
script for the video yesterday, my antique HP LaserJet 5P started
putting a gray line down the middle of the printout. I went off in
search of the spare toner cartridge that I'd bought back when the
current toner cartridge started showing signs of running out of toner.
I couldn't find the spare toner cartridge anywhere. Barbara searched,
but was also unsuccessful.
She finally checked my page, and
found that I'd ordered that spare toner cartridge from LaserMonks back
in 2003 (!). The toner cartridge that's currently in the printer is
only the second cartridge I've installed. I bought the printer about 13
years ago, so I'm getting an average of 6.5 years per toner cartridge.
I
was about to give up the search and order a new toner cartridge when I
realized that buying a new cartridge for a 13-year-old printer might
not be the best idea. After all, if this toner cartridge lasts as long
as the first two did, the printer would be about 20 years old when the
toner runs out. As reliable as the HP LaserJet 5P has been, it's
probably time to retire it.
I checked the Costco web site and found the Brother HL-5250DN for $230. Linux supports it.
It has an Ethernet interface, and does duplex printing. And Costco
currently has it on sale on their web site for $180, including
shipping, which is less than three times the price of just a new
cartridge for the old printer. So I'll order an HL-5250DN today.
08:20
- UPS showed up with my new National Optical 161-ASC microscope yesterday. I assembled it on the kitchen table. Once I get a space cleared off on my office credenza/desk, it'll go there.
The
microscope is larger than I expected, and quite hefty. It stands
about 18 inches (45 cm) tall at the top of the vertical eyepiece and
weighs about 15 pounds (6.5 kg). I expected the fit and finish to
be of "good Chinese" quality, but just looking at the scope you might
assume it was Japanese.
The only minor nit I could pick is that
the eyepieces are secured with tiny Phillips setscrews, about the size
of an eyeglass screw. A thumbscrew would be better, or at least a
captive setscrew. As it was, I had an exciting time trying to get
the eyepieces secured. I dropped the first setscrew twice. Fortunately,
it didn't roll off the kitchen table, or it might have been lost
forever.
Barbara was concerned about my plan to keep the scope
in my office. I smoke there, and quite a bit of dust accumulates. Not
to worry, I told her. I'd ordered a separate dustcover from Home
Science Tools. As it turns out, that'll be a spare, because the scope
comes with a dustcover, which wasn't mentioned in the literature. In
fact, they list it as a separate, optional item.
Actually, there's a good
chance that I won't be using either dustcover. I'll probably leave the
scope set up with one of our Pentax DSLRs perched on top of it, in
place of the vertical eyepiece. I think that assembly will be too large
to be covered by the standard dustcover. I'll probably end up using a
kitchen trash bag.
I
ordered the Brother HL-5250DN from Costco on-line yesterday. It shipped
last night. Before I ordered it, I checked on-line reviews at various
review sites as well as at Newegg.com and Amazon.com. This printer gets
extremely favorable reviews, including among people who've used it for
a year or more, so I think I'll be happy with it.
Several of the
reviewers and several of my own readers pointed out that the Brother
has a replaceable drum unit that, at $142, costs nearly as much as the
printer itself. I don't think that'll be an issue. The drum unit is
rated for 25,000 pages. I printed a grand total of 8,000 or 9,000 pages
on the HP LaserJet 5P in 13 years, so on that basis if the drum unit
reaches its rated lifetime it'd be good for about 40 years.
13:46
- Well, that was quick. I ordered the Brother HL-5250DN printer from Costco on-line yesterday at 9:36 a.m. UPS just delivered it.
09:15
-
I now have a microscopy station set up on the secondary desk in my
office, which sits to the left of my main desk. First light came
yesterday, when I popped a prepared slide into the slide holder and
turned on the lamp. The image was bright, sharp, and had very high
contrast. I'm quite pleased.
When Barbara got home from work
yesterday, I had her look through the microscope at the small, circular
algae. She asked what she was looking at, and I told her it was a
galaxy field in Coma Berenices. I narrowly avoided being hit.
Now
I need to figure out how to shoot images through the microscope. I want
to shoot prime-focus images, by connecting one of our Pentax DSLR
cameras to the microscope eyepiece tube without using either an
eyepiece or a camera lens. That is, there would be nothing but air
between the digital camera sensor and the microscope objective lens. I
need to figure out what I need in the way of adapter(s) to mount the
camera on the vertical eyepiece tube and get it at the right distance
to have the image in focus on the sensor.
I'm concerned about
back-focus distance. Obviously, with an eyepiece in place in the
eyepiece tube, the image plane is at the optical center of the eyepiece
itself. If I mount a camera on top of that eyepiece tube, that puts the
camera sensor several centimeters farther away, so I may need a shorter
eyepiece tube. Alternatively, I could (carefully) allow the eyepiece
tube to project through the adapter, putting it closer to the sensor. I
guess I'll call National Optical tech support and see what they
recommend.
08:30
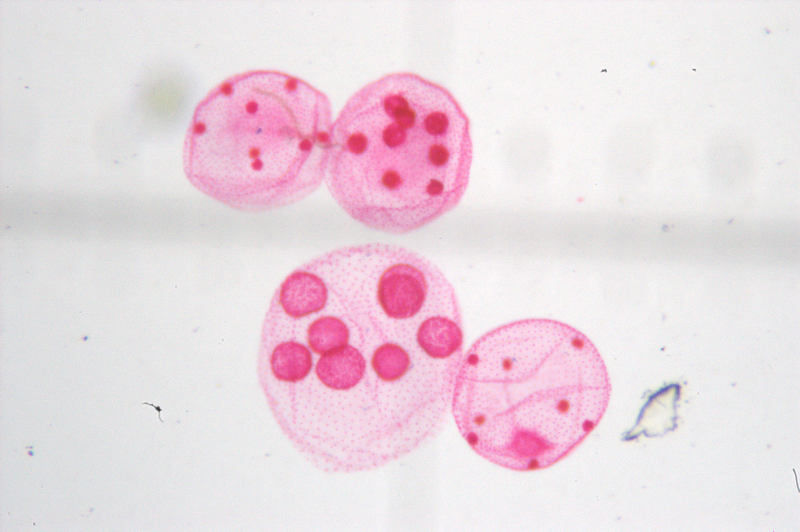
-
I spent some time yesterday playing around with imaging through the
microscope. I tried using a point-and-shoot camera held up to the
eyepiece, but it was very difficult to get it aligned properly. So then
I tried holding one of our Pentax DSLR bodies sans lens up to the
eyepiece. Here's what I got. The focus isn't great, but that's because
I was trying focus with one hand while holding the camera body with the
other. Just as I'd get the focus properly set, I'd let the camera body
move just a bit and the focus would be off again.
If I had the camera body mounted firmly in a fixed position, this would be doable. To do that, I think I'm going to order the Microscope Tube Adapter and a T-adapter from Edmund.
Incidentally,
all the dust and other crap is actually on the slide itself. However,
that light gray horizontal shadow through the center of the image is
actually a reticle, which is in sharp focus visually. Apparently, it's
at a different plane of focus than the slide, so it doesn't come to
focus simultaneously. That's not a problem, because this was shot
through the diagonal eyepiece on the front of the scope, which is used
visually. The vertical eyepiece, which I'll use for imaging, has no
reticle.
08:22
-
I ordered the microscope adapter tube and T-mount ring yesterday from
Edmund Scientific. I told the young woman who took my order that this
might be an all-time world record. When she asked why, I explained that
the last time I'd ordered anything from Edmund Scientific was 43 years
ago, in 1965, when I ordered a telescope kit with the materials to
grind my own mirror.
When
Barbara arrived home from work yesterday, she was stunned when I
announced that I'd gone out shopping on my own. It hasn't been 43 years
since I did that, but it's certainly not even an annual event. I think
the last time I did it was back in Bush's first term. No, come to think
of it, I did make a trip to Walgreen's some months ago to get some
stuff I needed for the home chem lab book. At any rate, I went to Ace
Hardware to buy a soil testing kit, of all things. Here's why.
From: Robert Bruce Thompson
To: Mary Chervenak, Paul Jones, Brian Jepson, Mindy Bedrossian, Barbara Thompson
Date: Thu Mar 20 16:53:57 2008
Re: [Home Forensics] Boy, am I cunning
I'm
working right now on the Soil Analysis chapter. I had just started
stubbing out one of the lab sessions I planned to include, Quantitative
Analysis of Phosphate Content, when I was struck by a cunning plan.
Although
yardwork is foreign to me, I know that soil test kits are available. So
I checked on-line, and sure enough I found a consolidated testing kit
for pH, nitrogen (presumably as nitrate), phosphorus (presumably as
phosphate), and potassium that uses visual colorimetry to give
quantitative results for those ions. So I ran over to Ace Hardware and
bought a kit for $16 plus tax. It's enough to do ten tests each for pH
and the three analytes.
You
get four little color-coded plastic test chambers, each with 10
color-coded capsules. You soak the test sample in five times its volume
of water, allow it to settle, and then fill up a test chamber with the
clear solution. You break one test capsule into the test chamber, allow
it to react for 10 minutes and then compare the color to a transparent
color chart right next to the reaction chamber. That chart is labeled:
0 - Depleted
1 - Deficient
2 - Adequate
3 - Sufficient
4 - Surplus
I'm
not sure how "Adequate" and "Sufficient" differ, but it's a for-sure
quantitative test. Being me, instead of using the chambers I'll
probably calibrate a test solution against standardized samples of
nitrate, phosphate, and potassium, but either way it'll give valid and
comparable results. Not bad for $16.
Now all I need to do is figure out what's in the nitrogen (pink), phosphorus (blue), and potassium (orange) capsules.
00:00
-
00:00
-
Copyright
© 1998,
1999, 2000, 2001, 2002, 2003, 2004, 2005, 2006, 2007, 2008 by Robert
Bruce
Thompson. All
Rights Reserved.